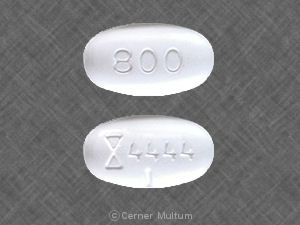
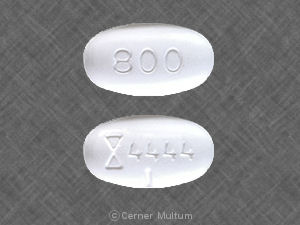
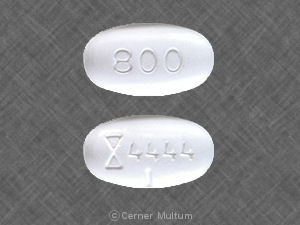
It is illegal in USA, Do not do it Neurontin is prescription and you must get a US licensed doctor to prescribe you a prescription…
Gabapentin is a non controlled substance. You can legally buy Gabapentin Online if you have a US Licensed doctor prescription. USAhealthstore.com has online doctor to…
Gabapentin capsules, tablets, and oral solution are used along with other medications to help control certain types of seizures in people who have epilepsy. Gabapentin…
Neurontin® (Gabapentin ) is another Epilepsy medication that is being used as an effective preventive or prophylactic Migraine regiment. As Migraine and Epilepsy are Related…
What is Gabapentin ? Gabapentin capsules, tablets, and oral solution are used along with other medications to help control certain types of seizures in people…
Neurontin is the trade name for the generic drug gabapentin. It is useful as an anti-epileptic drug and as an analgesic, particularly for pain of…
Muscle relaxant is a term usually used to refer to skeletal muscle relaxants (drugs), which act on the central nervous system (CNS) to relax muscles.…
What is Gabapentin? Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It affects chemicals and nerves in the body that are involved in the cause of…
Gabapentin capsules, tablets, and oral solution are used along with other medications to help control certain types of seizures in people who have epilepsy. Gabapentin…
Neuropathic pain is a chronic nerve pain that can significantly impair a patient’s functioning and quality of life. Neuropathic pain results from damaged nerves…